A 400-g block of iron at 400°C is dropped into a calorimeter containing 60 g of water at 30°C. How much steam is produced? The latent heat of vaporization of water is 22.6 × 105 J/kg
The average specific heat of iron over this temperature range is 560 J/(kg K). A) 22 g
B) 33 g
C) 42 g
D) 54 g
E) 59 g
A
You might also like to view...
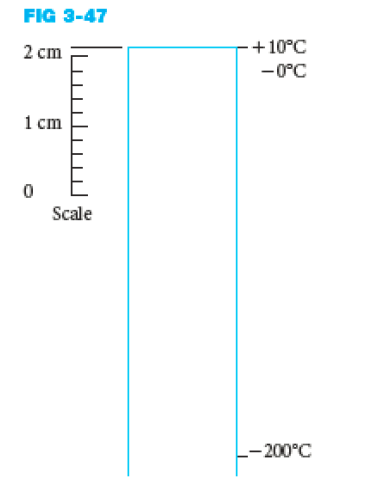
A large spherical tank is filled with liquid nitrogen at -200ºC. Using an approximation that the elements of the sphere are infinite walls with initial temperature of 10ºC, when the surroundings are at 10ºC as shown, predict the temperature through the wall after 5 minutes using graphical techniques. The tank is 1% carbon steel, 2 cm thick, and the convective heat transfer coefficients are 40 W/m^2 K for air and 60 W/m^2 K for the nitrogen. Also determine the time before the outside wall reaches 5ºC.
Carburetors in NASCAR race cars make use of Bernoulli's principle
a. True b. False Indicate whether the statement is true or false
Magnetic Poles: A straight bar magnet is initially 4 cm long, with the north pole on the right and the south pole on the left. If you cut the magnet in half, the right half will
A. contain only a north pole. B. contain a north pole on the right and a south pole on the left. C. contain only a south pole. D. no longer contain any poles.
The primary reason for the high specific heat of polymers is their low monomer _____________.
Fill in the blank(s) with the appropriate word(s).