The potential energy for the formation of a hydrogen molecule can be approximated by U = -A + BR + C/R, where R is the nuclear separation. Given that A = -78.5 eV, B = 2.57 × 1011 eV/m , and C = 1.44 × 10-9 eV?m
At what nuclear separation is the potential energy a minimum? A)
1.9 × 10-11 m
B)
3.7 × 10-10 m
C)
7.5 × 10-11 m
D)
2.9 × 10-11 m
E)
5.1 × 10-10 m
C
You might also like to view...
Newton never knew the numerical value of "his" universal gravitational constant G
Indicate whether the statement is true or false
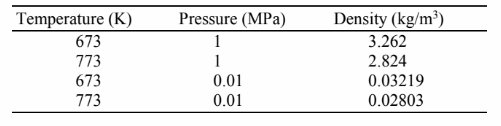
Using the standard steam tables, calculate the coefficient of thermal expansion, ?, from its definition for steam at 450°C and pressures of 10 kPa and 1 MPa. Then compare your results with the value obtained by assuming that steam is a perfect gas and explain the difference.
GIVEN
• Steam
• Temperature = 450°C = 723 K
FIND
The coefficient of thermal expansion at 10 kPa and 10 MPa from
(a) Standard Steam tables
(b) Perfect Gas Law
PROPERTIES AND CONSTANTS
From steam tables
The dynamo effect is believed to produce the ____________________ of the sun
Fill in the blank(s) with correct word
Beats: Two motors in a factory are running at slightly different rates. One runs at 825.0 rpm and the other at 786.0 rpm. You hear the sound intensity increase and then decrease periodically due to wave interference. How long does it take between successive instances of the sound intensity increasing?
A. 1.54 s B. 1.43 s C. 1.66 s D. 1.79 s