System 1 is 4.20 moles of a monatomic ideal gas held at a constant volume as it undergoes a temperature increase. System 2 is 4.20 moles of a diatomic ideal gas held at constant volume as it also undergoes a temperature increase. The thermal energy absorbed by System 1 is Q1, and the thermal energy absorbed by System 2 is Q2 as both systems undergo a temperature increase of 76.0 K. What is the
ratio Q1/Q2?
a. 1.00
b. 0.600
c. 1.67
d. The volumes of the systems need to be known before the ratio can be found.
e. 2.50
b
You might also like to view...
What is the direction of 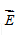 at point Q?
at point Q?
A. A B. B C. C D. D E. E F. F T. Zero
Which requires the most amount of work by the brakes of a car?
A) slowing down from 100 km/h to 70 km/h B) slowing down from 70 km/h to a stop C) equal amounts for both
Two ice skaters suddenly push off against one another starting from a stationary position. The 45-kg skater acquires a speed of 0.375 m/s relative to the ice. What speed does the 60-kg skater acquire relative to the ice?
A) 0.50 m/s B) 0.28 m/s C) 0.38 m/s D) 0.75 m/s E) 0.00 m/s
A hunter on level ground fires a bullet at an angle of 10 degrees below the horizontal while simultaneously dropping another bullet from the level of the rifle. Which bullet will hit the ground first?
A) the one dropped B) the one fired C) both hit at the same time.