Assuming the liquid is an ideal solution, determine the bubble point pressure, and determine whether the first bubble of vapor contains more product (C+D) or more reactant (A+B).
A reaction A+B?C+D occurs in the liquid phase. A vessel contains a liquid at P=0.5 MPa and T=298.15 K. The vessel initially contains 10 moles of A and 10 moles of B, but the reaction is allowed to reach equilibrium at P=0.5 MPa and T=298.15 K. After the reaction reaches equilibrium, the pressure is reduced isothermally until the first bubble of vapor forms. The equilibrium constant for the reaction is 0.8. Vapor pressures are 0.120MPa for compound A, 80kPa for compound B, 0.150MPa for compound C and 70kPa for compound D.
A. 0.105 MPa, product
B. 0.105 MPa, reactant
C. 0.165 MPa, product
D. 0.165 MPa, reactant
E. None of these
A. Incorrect. You have calculated the pressure correctly, but you need to take another look at the extent of reaction.
B. Correct. Using the definition of extent of reaction from the stoichiometric table you find the fractions of the four compounds. Using Raoult’s Law you get the bubble point pressure.
C. Incorrect. Review Raoult’s Law for the bubble point calculation.
D. Incorrect. Review Raoult’s Law for the bubble point calculation.
E. Incorrect. One answer is accurate.
You might also like to view...
En la Figura 2, la resistencia total del circuito es de _____ ohmios.
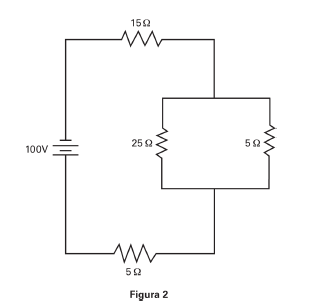
a. 19,18
b. 24,17
c. 30,08
d. 50
The addition of a window air conditioner of ____ size may overload a circuit.
A. medium B. any C. small D. large
The buttress thread is commonly used in situations where tubular features are screwed together and lateral forces are exerted in one direction.
Answer the following statement true (T) or false (F)
Which standards body deals with user-premises equipment and satellite communications?
A. IAB B. TIA C. IETF D. ISCO