Uncertainty Principle Using Momentum and Position: If you confine an electron to a box and know that the uncertainty in the electron's speed is 65 m/s, what is the smallest length that the box could have? (melectron = 9.11 × 10-31 kg, h = 6.626 × 10-34 J ? s)
A. 1.1 × 10-5 m
B. 1.8 × 10-6 m
C. 5.8 × 10-6 m
D. 1.8 × 10-5 m
E. 8.4 × 10-6 m
Answer: B
You might also like to view...
Spectroscopic parallax denotes the apparent shift in the position of a star when observed form different positions of a source
Indicate whether the statement is true or false
If VA?VB = 50 V, how much energy is stored in the 36-?F capacitor?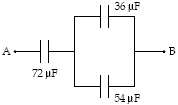
A. 50 mJ B. 28 mJ C. 13 mJ D. 8.9 mJ E. 17 mJ
The values of n and ? for a 4f subshell are
A) n = 4, ? = 4. B) n = 4, ? = 3. C) n = 3, ? = 3. D) n = 4, ? = 2. E) n = 3, ? = -3.
A camera lens having a 50-mm focal length is set to f/4.0. What is the minimum spacing of two objects located 12 m from the lens if the objects are just barely resolved in the image using light of wavelength 500 nm?
A) 4.9 × 10-5 m B) 0.024 mm C) 0.59 mm D) 4.72 mm E) 1.66 mm