Solve the problem.The hydrogen potential, pH, of a substance is defined by 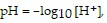 where the hydrogen ion concentration, [H+], is measured in moles per liter. Find the hydrogen ion concentration of a solution whose pH is 7.4.
where the hydrogen ion concentration, [H+], is measured in moles per liter. Find the hydrogen ion concentration of a solution whose pH is 7.4.
A. 3.98 × 10-8 moles per liter
B. 0.86923172 moles per liter
C. 2.51 × 107 moles per liter
D. -0.8692317 moles per liter
Answer: A
Mathematics
You might also like to view...
Graph the equation.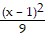 +
+ 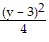 = 1
= 1
A. 
B. 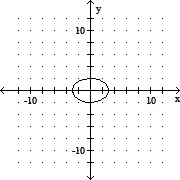
C. 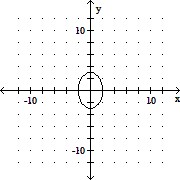
D. 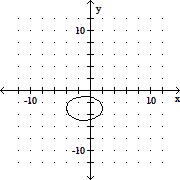
Mathematics
Add. Do not use a number line except as a check.-9.2 + 5.4
A. -14.6 B. -3.8 C. 14.6 D. 3.8
Mathematics
Solve the problem.Write the algebraic equation which can be used to find the exact solution of 
Fill in the blank(s) with the appropriate word(s).
Mathematics
Solve the percent of change problem.In a local election, 35,200 people voted. This was an increase of 12% over the last election. How many people voted in the last election? Round to the nearest whole number when necessary.
A. 30,976 people B. 39,424 people C. 31,429 people D. 40,000 people
Mathematics