Use the fact that the quantity of a radioactive substance after t years is given by 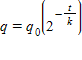 , where
, where 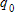 is the original amount of radioactive material and k is its half-life (the number of years it takes for half the radioactive substance to decay). The half-life of carbon-14 is 5,600 years. Find the amount of carbon-14 remaining after 8,000 years if
is the original amount of radioactive material and k is its half-life (the number of years it takes for half the radioactive substance to decay). The half-life of carbon-14 is 5,600 years. Find the amount of carbon-14 remaining after 8,000 years if
src="https://sciemce.com/media/3/ppg__cognero__Chapter_00_Algebraic_Concepts__media__b1c3d0cf-1972-465c-8704-797aabfd62a9.PNG" class="wirisformula" align="middle" style="vertical-align: middle;" data-wiris-created="true" varid="variable_id_field" variablename="impvar_5aee58072a364207822d3225a" />. Round your answer to four decimal places if required.
?
A. 24.9307 g
B. 65.7924 g
C. 0.0019 g
D. 15.0457 g
E. 20.25 g
Answer: D
Mathematics
src="https://sciemce.com/media/3/ppg__cognero__Chapter_00_Algebraic_Concepts__media__b1c3d0cf-1972-465c-8704-797aabfd62a9.PNG" class="wirisformula" align="middle" style="vertical-align: middle;" data-wiris-created="true" varid="variable_id_field" variablename="impvar_5aee58072a364207822d3225a" />. Round your answer to four decimal places if required.
?
A. 24.9307 g
B. 65.7924 g
C. 0.0019 g
D. 15.0457 g
E. 20.25 g
Answer: D
Mathematics
You might also like to view...
Decide whether or not the fractions are equivalent.- 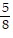 and -
and - 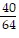
A. Not equivalent B. Equivalent
Mathematics
Write the following proportions.$36 is to 32 bottles as $63 is to 56 bottles
A. 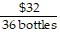 =
= 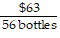
B. 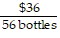 =
= 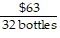
C.  =
= 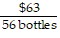
D. 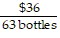 =
= 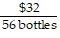
Mathematics
Solve the equation.10y = 5y + 4 + 4y
A. -4 B. 4 C. -40 D. 40
Mathematics
Find csc ? if 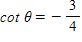
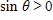
What will be an ideal response?
Mathematics