Solve the problem.The pH of a solution is defined as pH = -log[H+], where [H+] is the concentration of hydrogen ions in the solution. The pH of pure water is 7, while the pH of orange juice is about 4. How much greater is the concentration of hydrogen ions in orange juice than in pure water?
A. 100 times greater
B. 1000 times greater
C. 3 times greater
D. 10 times greater
Answer: B
You might also like to view...
Reverse the order of integration and then evaluate the integral.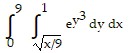
A. 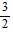 (e - 1)
(e - 1)
B. 3(2e - 1)
C. 3(e - 1)
D. 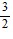 (2e - 1)
(2e - 1)
Solve.Find the midpoint of the line segment formed by joining the points P1 = (9, 0) and P2 = (-4, 5).
A. 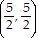
B. (5, 5)
C. (13, -5)
D. 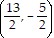
Solve the equation.x(x + 18) = 0
A. {-18, 1} B. {-18, -1} C. {0, 18} D. {-18, 0}
Solve.The equation that represents the proper traffic control and emergency vehicle response availability in a small city is 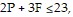 where P is the number of police cars on active duty and F is the number of fire trucks that have left the firehouse in response to a call. In order to comply with staffing limitations, the equation
where P is the number of police cars on active duty and F is the number of fire trucks that have left the firehouse in response to a call. In order to comply with staffing limitations, the equation 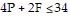 is appropriate. The number of police cars on active duty and the number of fire trucks that have left the firehouse in response to a call cannot be negative, so
is appropriate. The number of police cars on active duty and the number of fire trucks that have left the firehouse in response to a call cannot be negative, so 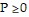
style="vertical-align: -4.0px;" /> and  Graph the regions satisfying all the availability and staffing requirements, using the horizontal axis for P and the vertical axis for F. If 4 police cars are on active duty and 5 fire trucks have left the firehouse in response to a call, are all of the requirements satisfied?
Graph the regions satisfying all the availability and staffing requirements, using the horizontal axis for P and the vertical axis for F. If 4 police cars are on active duty and 5 fire trucks have left the firehouse in response to a call, are all of the requirements satisfied?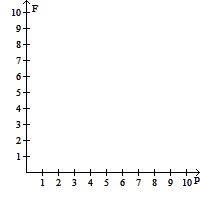
A. 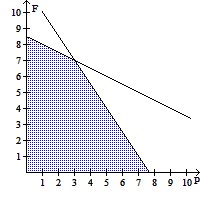 ; Yes
; Yes
B. 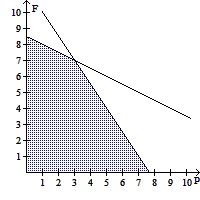 ; No
; No
C. 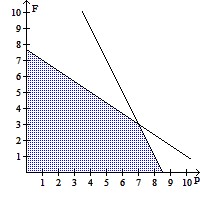 ; No
; No
D. 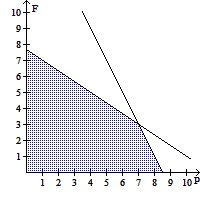 ; Yes
; Yes