Calculate the normality of the following solutions:
a. 36.5 g/L hydrochloric acid [HCl]
b. 80 g/L sodium hydroxide [NaOH]
c. 9.8 g/L sulfuric acid [H2SO4]
d. 9.0 g/L acetic acid [CH3COOH]
What will be an ideal response?
a. 36.5 g/L hydrochloric acid [HCl]
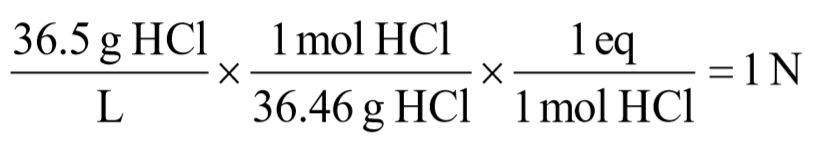
b. 80 g/L sodium hydroxide [NaOH]
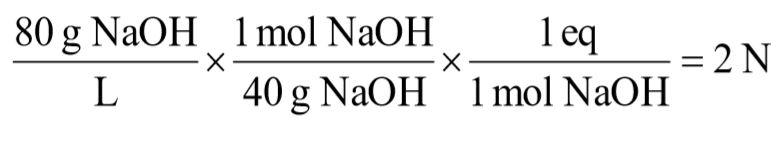
c. 9.8 g/L sulfuric acid [H2SO4]
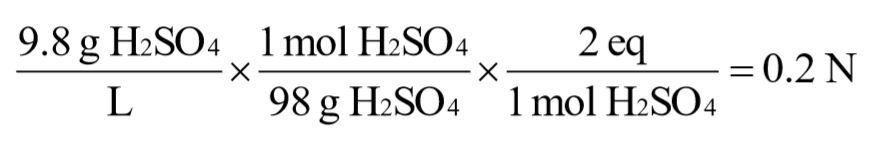
d. 9.0 g/L acetic acid [CH3COOH]
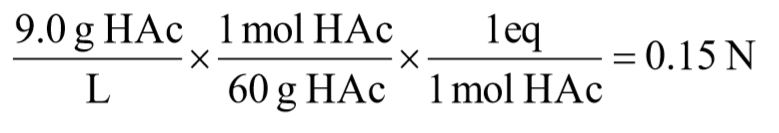
You might also like to view...
What is a datum?
What will be an ideal response?
Use PSpice to find I?, I?, and I? in the circuit of Fig. 13.139.
 figure 1.png)
When an air-cooled split system operates at low ambient temperatures:
A) System efficiency is increased because of the extra cooling effect of the colder air. B) Head pressure increases because of the inverse relationship between ambient temperature and condenser temperature. C) Low ambient controls are necessary for safe operation. D) The decreased air density reduces the airflow over the condenser.
When making a knurled handle on a hammer, a lathe should be used.
Answer the following statement true (T) or false (F)