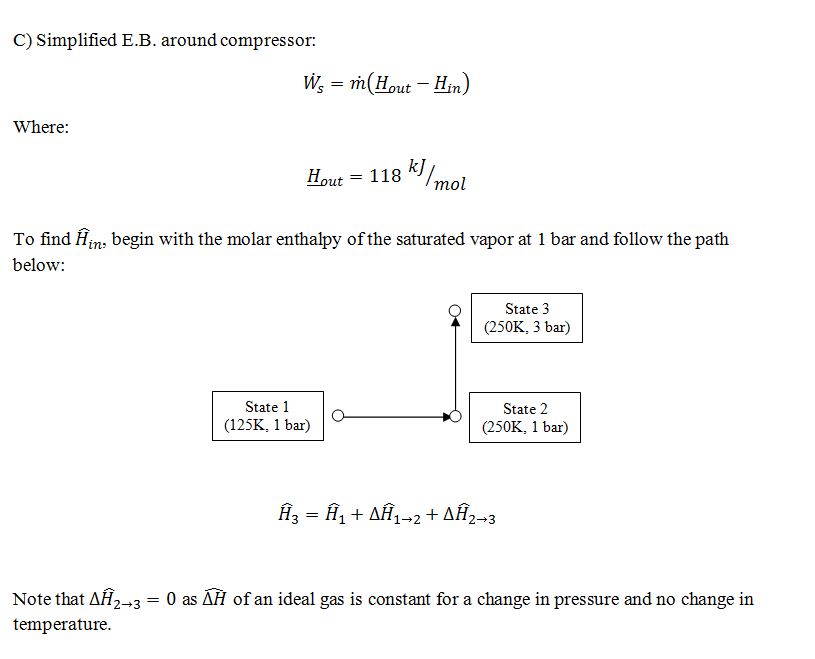
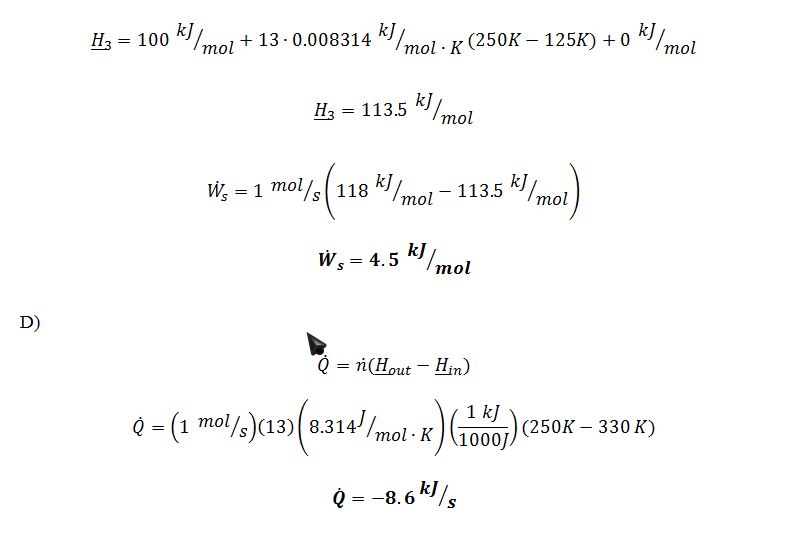
A) What is the molar enthalpy of saturated liquid at P=1 bar? B) What is the temperature of the 0.8 mol/sec of low-pressure vapor when it leaves the heat exchanger that is described in step 4? C) One of the compressors (step 5) takes in 1 mol/sec of vapor at P=3 bar and T=250 K, and emits it at P=10 bar and T=350 K. (These are the actual conditions in and out of the actual compressor.) What is the rate at which work is added to this compressor? D) One of the heat exchangers takes in 1 mol/sec of gas at P=3 bar and T=330 K, and emits it at P=3 bar and T=250 K. How much heat is removed in this heat exchanger?
1. A compound is very volatile and can only be liquefied at extremely low temperatures. At pressures of 3 bar and below, it can be modeled as an ideal gas with a constant Cp*=13R. The following list contains some additional data regarding the compound:
Boiling temperature at 1 bar = 125 K
Molar enthalpy of saturated vapor at 1 bar: HV = 100 kJ/mol
Critical Pressure = 8 bar
Molar enthalpy of supercritical fluid at P=10 bar and various temperatures:
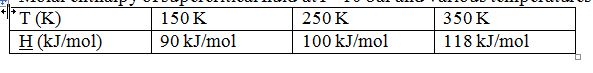
A steady-state Linde Liquefaction process for this compound works as follows:
1. One mole per second of supercritical fluid at P=10 bar and T=250 K enters a counter-current heat exchanger, where its temperature is lowered to 150 K.
2. The supercritical fluid leaving step 1 enters a flash chamber where it undergoes an adiabatic (Joule-Thompson) expansion to P=1 bar, and 20% of it liquefies.
3. The 0.2 mol/sec of saturated liquid at P=1 bar is removed as product.
4. The 0.8 mol/sec of saturated vapor at P=1 bar is returned to the heat exchanger of step 1, where it acts as the coolant for the entering supercritical fluid.
5. The vapor from step 4 is mixed with 0.2 mol/sec of fresh feed, and the mixed stream enters a series of compressors and heat exchangers which return the stream to 10 bar and 250 K.
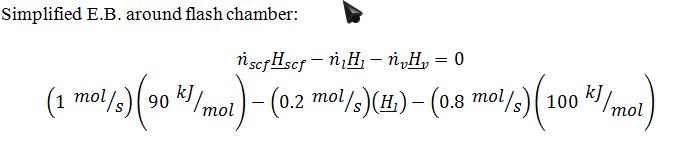
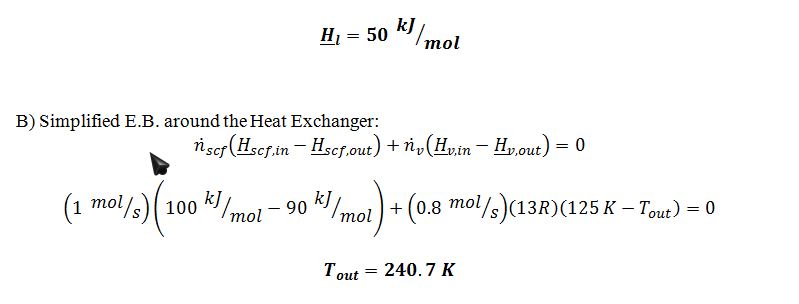
You might also like to view...
If you are using annular ring nails to install 5/8" drywall, the length of the nail should be at least _____.
a. 1" b. 1-1/4" c. 1-3/8" d. 1-1/2"
The degree to which forces in the environment change over time is known as
a. technological change. b. economic change. c. sociocultural change. d. environmental change. e. political change.
What was one of the legacies of the Dutch in the colony of New York?
A. a spirit of fierce individualism B. religious intolerance C. an autocratic spirit D. liberal values E. preference for urban development
A characteristic of water vapor, but not other states of water, is:
A) Water vapor is compressible. B) Water exerts equal pressure on the sides of a container in which it is placed. C) Water molecules move about freely. D) Small changes in the volume of water occur with changes in temperature.