Three moles of an ideal gas with a molar heat capacity at constant volume of 4.9 cal/(mol?K) and a molar heat capacity at constant pressure of 6.9 cal/(mol?K) starts at 300 K and is heated at constant pressure to 320 K,
then cooled at constant volume to its original temperature. How much heat flows into the gas during this two-step process?
A)
710 cal
B)
-720 cal
C)
0 cal
D)
120 cal
E)
-120 cal
D
You might also like to view...
In which of the following cases would you feel weightless?
A) while falling from a roof B) while parachuting from an airplane C) while accelerating downward in an elevator D) while walking on the Moon
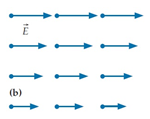 Which of the fields are physically impossible and why?
Which of the fields are physically impossible and why?
A. This field violates Faraday’s law. B. This field violates Gauss’s law. C. This field violates both of the above laws. D. This field is physically possible.
Essay? Explain what a web-based form is, how companies use them, and how users use them.
What will be an ideal response?
Granulation is caused by:
A) shock waves in the corona. B) rising and falling currents of gas in and just below the photosphere. C) the solar wind flowing away from the corona. D) the temperature variability in the chromosphere, which causes sudden heating.