The mixture is stable and forms one liquid phase.
Consider the figure that provides the molar Gibbs free energy of mixing as a function of composition for a binary mixture.
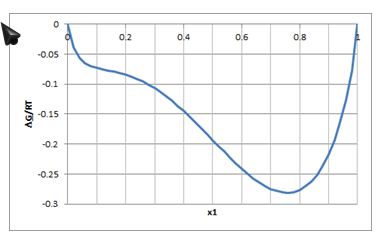
If the overall composition of the mixture is 30% of component 1, is the mixture stable? If not, what are estimates of the compositions of the liquid phases that are in equilibrium?
Incorrect. The mixture is NOT stable in one phase. Draw the tangent line and try again.
You might also like to view...
On 100 different days, a traffic engineer counts the number of cars that pass through a certain intersection between 5 P.M. and 5:05 P.M. The results are presented in the following table.
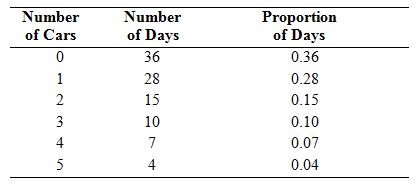
a. Let X be the number of cars passing through the intersection between 5 P.M. and 5:05 P.M. on a randomly chosen day. Someone suggests that for any positive integer x, the probability mass function of X is 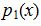 =(0.2)(0.8)x. Using this function, compute P(X=x) for values of x from 0 through 5 inclusive.
=(0.2)(0.8)x. Using this function, compute P(X=x) for values of x from 0 through 5 inclusive.
b. Someone else suggests that for any positive integer x, the probability mass function is 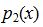 =(0.4)(0.6)x. Using this function, compute P(X=x)for values of x from 0 through 5 inclusive.
=(0.4)(0.6)x. Using this function, compute P(X=x)for values of x from 0 through 5 inclusive.
c. Compare the results of parts (a) and (b) to the data in the table. Which probability mass function appears to be the better model? Explain.
d. Someone says that neither of the functions is a good model since neither one agrees with the data exactly. Is this right? Explain.
The most profound changes in the future will occur in
What will be an ideal response?
The _____ breed of geese exists in two varieties
a. African c. Embden b. Pilgrim d. Chinese
Electrical drawings are typically drawn with all components de-energized.
Answer the following statement true (T) or false (F)