Solve the problem.The pressure of a gas varies jointly as the amount of the gas (measured in moles) and the temperature and inversely as the volume of the gas. If the pressure is 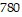 kiloPascals (kPa) when the number of moles is 4, the temperature is
kiloPascals (kPa) when the number of moles is 4, the temperature is 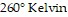 , and the volume is
, and the volume is  , find the pressure when the number of moles is 2, the temperature is
, find the pressure when the number of moles is 2, the temperature is 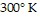 ,
,
and the volume is 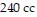 .
.
A. 1,800 kPa
B. 900 kPa
C. 840 kPa
D. 1,920 kPa
Answer: B
Mathematics
You might also like to view...
Factor the polynomial using the greatest common binomial factor.x(y2 - 6) + 11(y2 - 6)
A. 11x(y2 - 6) B. (y2 - 6)(x - 11) C. (xy2 - 6x) + (11y2 - 66) D. (y2 - 6)(x + 11)
Mathematics
Factor the difference of squares.9s5 - 25st4
A. (3s2 + 5st2)(3s2 - 5st2) B. s(3s2 + 5t2)(3s2 - 5t2) C. s(3s2 + 5t2)(s + t)(s - t) D. 9s(s2 + t2)(s + t)(s - t)
Mathematics
Solve the absolute value inequality. Other than ?, use interval notation to express the solution set and graph the solution set on a number line.|3x - 8| - 3 > -9![]()
A. (-?, ?)![]()
B. 
![]()
C. 
![]()
D. ?![]()
Mathematics
Find the distance from P1 to P2.P1 = (0, 1, -1) and P2 = (-1, -3, 3)
A. 3
B. 4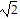
C. 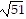
D. 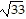
Mathematics