Using Henry’s constant data for a gas dissolved in a liquid, explain how you would determine the mole fraction of the gas dissolved in the liquid at the interface at a specified temperature.
What will be an ideal response?
Using Henry’s constant data for a gas dissolved in a liquid, the mole fraction of the gas dissolved in the liquid at the interface at a specified temperature can be determined from Henry’s law expressed as
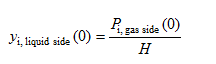
where H is Henry’s constant and Pi, gas side(0) is the partial pressure of the gas i at the gas side of the interface. This relation is applicable for dilute solutions (gases that are weakly soluble in liquids).
You might also like to view...
How is voltage tested if one side of the control transformer is grounded?
What will be an ideal response?
Copper wire sizes are measured in circular mils.
Answer the following statement true (T) or false (F)
In a profile description, soil horizons are normally labeled as #1, #2, #3, starting at the bottom of the profile
Indicate whether the statement is true or false
Schematic spring representations show ____.
A. major diameter; diagonal lines B. approximate representation of the coils using lines C. minor diameter and free length; diagonal lines D. free length and pitch; horizontal lines E. none of the above