Why is 2,4,5-trifluorophenol much more acidic than phenol?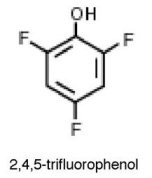
A. The fluorines destabilize the benzene ring and thus give the structure more acidic characteristics.
B. False! 2,4,5-trifluorophenol is actually not more acidic than phenol.
C. The fluorines have high electronegativities and help to stabilize the negative charges, which makes it even easier for the hydrogen to be lost.
D. The fluorines adjacent to the OH group are large and shield the hydroxyl group from making the solution basic.
Answer: C
You might also like to view...
An agreed-upon common language to facilitate communication on specific topics such as business is called a(n)
A) dialect. B) official language. C) language group. D) language family. E) lingua franca.
What type of clouds are most common in a hurricane?
A) nimbostratus B) cumulonimbus C) cirrus D) stratus
What type of plate boundary is most likely to generate a tsunami?
A. Convergent B. Transform C. Divergent
Currently Earth is ________ during the Northern Hemisphere winter.
A. at 16.5 degrees tilt B. at its average distance from the Sun C. closest to the Sun D. farthest from the Sun E. at 25.5 degrees tilt