If 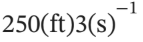 of air at 122(°F) and approximately atmospheric pressure is preheated for a combustion process to 932(°F), what rate of heat transfer is required?
of air at 122(°F) and approximately atmospheric pressure is preheated for a combustion process to 932(°F), what rate of heat transfer is required?
What will be an ideal response?
we are reminded that the gas constant is 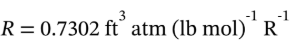 in the sort of
in the sort of
units used in this problem, so we can convert the volumetric flow rate to a molar flow rate using the ideal gas law (air at atmospheric conditions is very nearly an ideal gas).

To find the heat needed to heat the air at constant pressure from 122°F to 932 °F, we need to integrate the heat capacity over that temperature range
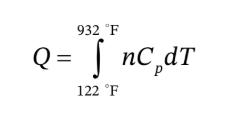
The ideal gas heat capacity for air is given in
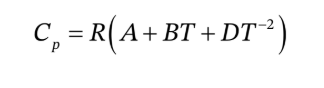
T must be in Kelvins for use in this expression, so we convert 122°F to 323.15 K and 932 °F to 773.15 K. We then have
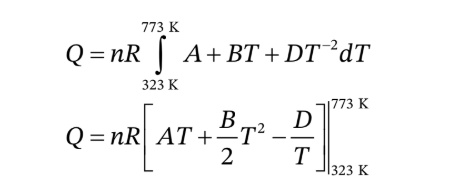
Putting in n = 0.59 lbmol 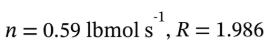 gives
gives 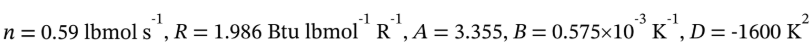
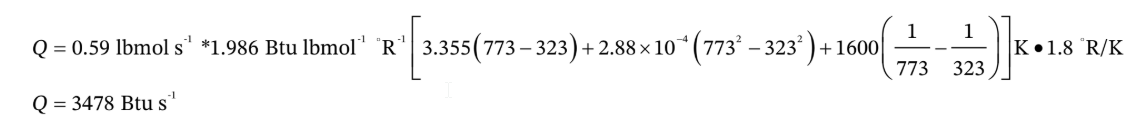
Note that because the heat capacity integral comes out with units of K, while we are using R with units of °R, we have to multiply by 1.8 °R/K.
You might also like to view...
Which of the following is an example of a drum mixer?
A. Washing machine B. Cement truck C. Kitchen mixer D. Garden hose
Growing plants from seeds is used to grow many of our important food crops as well as ornamental landscaping plants
Indicate whether the statement is true or false
What gauges are used for measuring duct pressure?
A) Compound gauges B) High pressure gauges C) Vacuum gauges D) Magnehelic gauges
One of the most common grounding systems used for protection against accidental electrical shock is the _____
A) two-wire system B) three-wire system C) four-wire system D) five-wire system