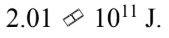A substance has a melting point of 20°C and a heat of fusion of 3.9 x 104 J/kg. The boiling point is 150°C and the heat of vaporization is 7.8 x 104 J/kg at a pressure of 1.0 atm
The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg?K), 1000 J/(kg?K), and 400 J/(kg?K), respectively. The quantity of heat required to raise the temperature of 3.80 kg of the substance from -61°C to 128°C, at a pressure of 1.0 atm, is closest to
A) 620 kJ.
B) 470 kJ.
C) 560 kJ.
D) 210 kJ.
E) 770 kJ.
A
You might also like to view...
A person weighs 155 pounds. What is the person's weight in Newtons?
A. 155 N B. 689 N C. 39.3 N D. 392 N
Which of the following methods describes the transit method for detecting extrasolar planets?
A) regular changes in the positions of the parent stars with respect to more distant stars as they move across the sky B) detection of Doppler shifts in the spectra of the parent stars C) detection of brightness changes in a star as a planet passes in front of it D) detection of reflected starlight
Electric field lines
A) are closer together the stronger the field. B) start on negative charges and end on positive charges. C) were invented by Isaac Newton. D) are perpendicular to the lines of force. E) were discovered by Franklin.
An 8000 kg satellite is launched from the surface of the earth, and injected into a circular orbit at an altitude of 100 km above the surface of the earth. The kinetic energy of the satellite in the circular orbit is 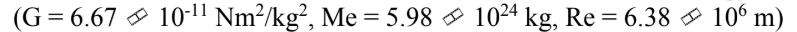
A. 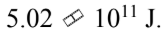
B. 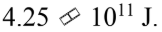
C. 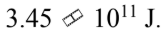
D. 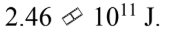
E.