The conversion of ammonium cyanide to urea is a second-order reaction. This means that the concentration C of ammonium cyanide at time t is given by 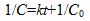 , where
, where 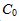 is the initial concentration and k is the rate constant. Assume the initial concentration is known to be 0.1 mol/L exactly. Assume that time can be measured with negligible uncertainty.
is the initial concentration and k is the rate constant. Assume the initial concentration is known to be 0.1 mol/L exactly. Assume that time can be measured with negligible uncertainty.
a. After 45 minutes, the concentration of ammonium cyanide is measured to be 0.0811 ± 0.0005 mol/L. Estimate the rate constant k, and find the uncertainty in the estimate.
b. Use the result in part (a) to estimate the time when the concentration of ammonium cyanide will be 0.0750 mol/L, and find the uncertainty in this estimate.
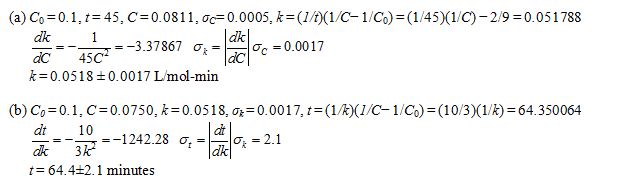
You might also like to view...
In a sound absorption type muffler, what is sonic energy converted to?
A. friction, then heat B. mechanical vibration C. pressure D. noise
When using the longest run method for sizing fuel gas piping, the required piping capacity must be ____________________ as each additional appliance is connected to the system.
Fill in the blank(s) with the appropriate word(s).
Annual plants deficient in P usually flower and mature more rapidly than P-sufficient plants
Indicate whether the statement is true or false
In the MATLAB Command Window, if you type the following commands, what would be the result?
scores = [80 90 50 70 80 60 65 95 70 40]; for i=1:1:10 if scores (i) <60 fprintf ('\t %g \t\t\t\t\t FAILING\n', scores (i)) end end