If you brought air at 32°C with 90% relative humidity into your house and cooled it down to 21°C, how many grams of water would condense per kilogram of air?
This question show how the relative humidity of warm, moist outside air changes when it is brought inside and cooled. People who live in hot, humid climates have to dehumidify their air to feel comfortable. As air cools, its relative humidity increases, making it feel muggy.
What will be an ideal response?
27.9 - 7.5 = 20.4 g/kg
You might also like to view...
Of the following, ________ is not a form of renewable energy
A) solar B) coal C) wind D) geothermal E) tidal
The ________ group of minerals is characterized by two parallel chains of silica tetrahedra in their structure.
A. pyroxene B. amphibole C. feldspar D. mica (biotite, muscovite, etc.) E. olivine
All the following are considered indoor air pollutants except ________
A) radon B) asbestos C) cigarette smoke D) pesticides E) sodium chloride
Dynamic forcing of upper air currents will create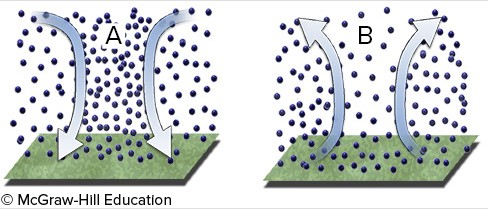
A. upper air convergence at B that results in surface high pressure. B. upper air convergence at B that results in surface low pressure. C. upper air convergence at A that results in surface high pressure. D. upper air divergence at A that results in surface high pressure.