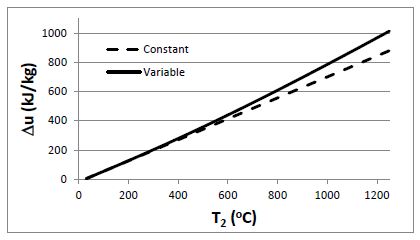
Air is initially at 25°C. Plot the change in specific internal energy of the air as it is heated to final temperatures ranging between 30°C and 1200°C, considering (a) the specific heats to be constant with cv = 0.718 kJ/kg-K, and (b) the specific heats to be variable
Given: T1 = 25°C
What will be an ideal response?
(a) For an ideal gas with constant specific heats, ?u = cv(T2 – T1) . Take cv = 0.718 kJ/kg-K
(b) For an ideal gas with variable specific heats. ?u = u2 – u1 .
The values for the specific internal energy can be found on-line or from air tables at specific temperatures.
u1 = 212.64 kJ/kg
Plotting the change in specific internal energy yields
You might also like to view...
The chief drawback in the use of ____ is the less-steady-arc characteristics and a considerable increase in weld spatter.
A. carbon dioxide B. argon C. oxygen D. helium
What is the best way to prevent avian tuberculosis?
What will be an ideal response?
The electron flow within an electrical circuit is known as ____.
A. voltage B. amperage C. resistance D. wattage
A "confined space" is defined as an area with limited access, capable of being entered to perform work, and _________.
A. located in a submarine B. not intended for employee occupancy C. always painted red D. below a fuel hopper