What would be the greatest effect on the ideal gas law if there is a slight attractive force between the molecules?
A)
At low temperatures, the pressure would be less than that predicted by the ideal gas law.
B)
At low temperatures, the pressure would be greater than that predicted by the ideal gas law.
C)
At high temperatures, the pressure would be greater than that predicted by the ideal gas law.
D)
At high temperatures, the pressure would be less than that predicted by the ideal gas law.
E)
There is no effect.
A
You might also like to view...
The diameter of a circle is doubled. By what factor is the area changed?
A. 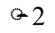
B. 2
C. 4
D. 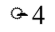
E. 1
A plane parallel plate capacitor has plates of 10 cm2 area that are 1.0 mm apart. At an instant when charge is being accumulated on the plates at a rate of 12 nC/s, the displacement current between the plates is
a. 1.06 × 10^?16 A. b. 1.2 × 10^?8 A. c. 8.85 × 10^?9 A. d. 1.00 A. e. 1.36 A.
The motion of a piston in an auto engine is simple harmonic. If the piston travels back and forth over a distance of 10 cm, and the piston has a mass of 1.5 kg, what is the maximum speed of the piston and the maximum force acting on the piston when the engine is running at 4 200 rpm?
A rock held above the ground has potential energy. As the rock falls, this potential energy is converted to kinetic energy. Finally, the rock hits the ground and stays there. What has happened to the energy?
A) The energy goes to producing sound and to heating the ground, rock, and surrounding air. B) The energy goes into the ground, and as a result, the orbit of the Earth about the Sun is slightly changed. C) The rock keeps the energy inside it in the form of mass-energy. D) It is transformed back into gravitational potential energy.