Air at 1200 kPa and 250°C fills a balloon with a volume of 2.85 m3. The balloon cools and expands until the pressure is 400 kPa. The pressure and volume follow a relationship given by PV1.3 = constant. Determine the work done by the air as it expands, and the final temperature of the air.
Given: P1 = 1200 kPa; T1 = 250°C = 523 K; V1 = 2.85 m3; P2 = 400 kPa; PV1.3 = constant
What will be an ideal response?
From the ideal gas law, m = P1V1/RT1
For air, R = 0.287 kJ/kg-K, so m = 22.78 kg
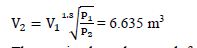
The moving boundary work for a polytropic process with n ? 1:
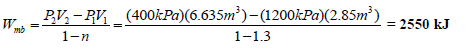
From the ideal gas law:
T2 = P2V2/mR = 406 K = 133oC
You might also like to view...
Intersecting walls that carry the load of floors and roofs of buildings must also be able to stretch or flex.
Answer the following statement true (T) or false (F)
The power diodes in the converter can be tested with an ____.
a. ohmmeter b. ammeter c. autometer d. R-meter
What color is printed on the labels of standard interrupting capacity circuit breakers?
a. White b. Black c. Red d. Blue
Why is it necessary to use special methods when recovering from Type III systems?
A) Most of these systems are very low pressure. B) Most of these systems utilize toxic chemicals. C) Most of these systems are very small-scale. D) Most of these systems are chillers.