Alkaloid salts are not very soluble in the organic solvent diethyl ether. What might happen to the free-base form of caffeine dissolved in diethyl ether if gaseous hydrogen chloride, HCl, were bubbled into the solution?
A) A second layer of water would form.
B) Nothing, and the HCl gas would merely bubble out of solution.
C) The diethyl ether insoluble caffeine salt would form as a white precipitate.
D) The acid/base reaction would release heat, which would cause the diethyl ether to start evaporating.
Answer: C
You might also like to view...
A 50-W light bulb is in a socket supplied with 140 V. What is the current in the bulb?
a. 0.36 A b. 2.8 A c. 90 A d. 7 000 A e. 3.8 A
Schwarzschild showed how the _______________ of the event horizon depends only on the mass of the black hole
Fill in the blank(s) with correct word
A tractor T is pulling two trailers, 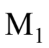 and
and 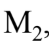 with a constant acceleration. T has a mass of 200 kg,
with a constant acceleration. T has a mass of 200 kg, 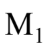 has a mass of 100 kg, and
has a mass of 100 kg, and 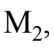 has a mass of 150 kg. If the forward acceleration is 0.60
has a mass of 150 kg. If the forward acceleration is 0.60 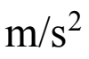 then the horizontal force on
then the horizontal force on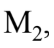 due to the attachment to
due to the attachment to 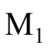 is
is
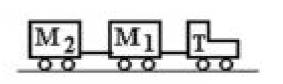
A. 270 N backward.
B. 270 N forward.
C. 150 N backward.
D. 150 N forward.
E. 90 N forward.
On the H-R diagram, red supergiants like Betelgeuse lie
A) at the bottom left. B) at the top left. C) at the top right. D) at the bottom right. E) They can't be plotted, for they are not main sequence.