A 60-gallon water heater is initially filled with water at 50°F. Determine how much energy (in Btu) needs to be transferred to the water to raise its temperature to 120°F. Evaluate the water properties at an average water temperature of 85°F
What will be an ideal response?What will be an ideal response?
A water heater is initially filled with water at 50?F. The amount of energy that needs to be transferred to the water to raise its temperature to 120?F is to be determined.
Assumptions 1 Water is an incompressible substance with constant specific. 2 No water flows in or out of the tank during heating.
Properties The density and specific heat of water at 85ºF from Table A-9E are:
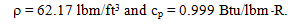
Analysis The mass of water in the tank is
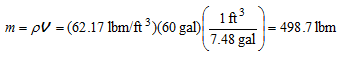
Then, the amount of heat that must be transferred to the water in the tank as it is heated from 50 to 120?F is determined to be
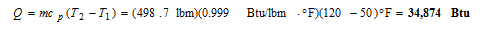
Discussion Referring to Table A-9E the density and specific heat of water at 50ºF are: ? = 62.41 lbm/ft3 and cp = 1.000 Btu/lbm?R and at 120ºF are: ? = 61.71 lbm/ft3 and cp = 0.999 Btu/lbm?R. We evaluated the water properties at an average temperature of 85ºF. However, we could have assumed constant properties and evaluated properties at the initial temperature of 50ºF or final temperature of 120ºF without loss of accuracy.
You might also like to view...
Which term best describes fish?
a. ectothermic c. heliothermic b. endothermic d. normothermic
All successful salespeople are ____________________ about their products
Fill in the blank(s) with correct word
The most effective way for an organization to succeed in the global marketplace is to consistently provide superior value to its customers.