What element is behaves as the oxidizing agent in the following equation and what element behaves as the reducing agent?
Sn2+ 2 Ag ? Sn + 2 Ag?
A) The tin ion, Sn2, is the oxidizing agent while silver, Ag, is the reducing agent.
B) The tin ion, Sn2+, is the reducing agent while silver, Ag, is the oxidizing agent.
C) The tin, Sn, is the reducing agent while silver ion, Ag?, is the oxidizing agent.
D) The tin, Sn, is the oxidizing agent while silver ion,Ag?, is the reducing agent.
Answer: A
You might also like to view...
Estimate the time required to heat the center of a 1.5-kg roast in a 163°C oven to 77°C. State your assumptions carefully and compare your results with cooking instructions in a standard cookbook.
GIVEN
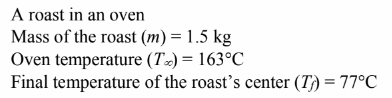
FIND
The time required to heat the roast
ASSUMPTIONS

SKETCH
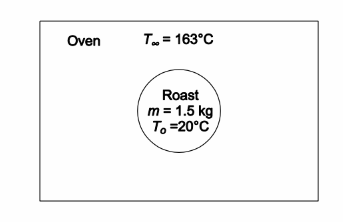
A part is to be made from the 4340 steel in Table 11.2. The part has a yield strength of 1515 MPa. This design requires that the calculated fracture stress must be at least twice as large as the yield stress, to ensure that fracture does not occur before yield. What is the minimum detectable crack length capability required of the NDT equipment to ensure this condition?
What will be an ideal response?
The water hole has very high background interference
Indicate whether the statement is true or false
The rapid fluctuations of quasars show that the energy emitting portions of these objects must be very small
Indicate whether the statement is true or false