Two atoms have the same number of neutrons but different numbers of protons. These two atoms
A. have the same atomic mass.
B. will exhibit different chemical properties.
C. have the same number of electrons.
D. are isotopes of each other.
Answer: B
You might also like to view...
Consider a set of electrons with an initial spin state 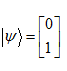 If we send these electrons into an SGx device, what is the probability a given electron will be determined to have its spin anti aligned with the +x direction?
If we send these electrons into an SGx device, what is the probability a given electron will be determined to have its spin anti aligned with the +x direction?
A. 0
B. 1/4
C. 1/2
D. 
E. 1
All four of Jupiter's big moons, like most moons in the solar system, revolve clockwise (retrograde) around their planet's equator
Indicate whether the statement is true or false
The kinetic energy of a proton is 80% of its total energy. What is the momentum in kg?m/s of the proton?
A) 5.0 × 10-19 kg?m/s B) 2.5 × 10-19 kg?m/s C) 5.0 × 10-18 kg?m/s D) 2.5 × 10-18 kg?m/s E) 1.5 × 10-19 kg?m/s
100 g of liquid nitrogen at its boiling point of 77 K is stirred into a beaker containing 600 g of 15°C water. If the nitrogen leaves the solution as soon as it turns to gas, how much water freezes? The heat of vaporization of nitrogen is 48 cal/g and that of water is 80 cal/g
a. none c. 68 g b. 29 g d. 109 g