In a reactor, the gas phase reaction A ? B is at equilibrium with yA = 0.75 and yB = 0.25. An inert gas is added to the mixture, but no A or B is added or removed. Then the system is allowed to reach a new equilibrium at the same T and P as the original equilibrium. Both equilibrium states can be modeled as ideal solutions. Which of the following correctly describes the effect of adding the inert on the system?
A. The reaction will shift to the right, forming more moles of B than in the original equilibrium.
B. The reaction will shift to the left, forming more moles of A than in the original equilibrium.
C. The numbers of moles of A and B will be the same as in the original equilibrium.
D. Answers A and B are both possible but the correct one cannot be determined from the given information.
E. The scenario is impossible because adding inert gas will inevitably increase the pressure.
A. Incorrect. The equilibrium expression for this ideal gas system is K = yB/yA. Adding an inert gas will lower both mole fractions, but will not change their ratio.
B. Incorrect. The equilibrium expression for this ideal gas system is K = yB/yA. Adding an inert gas will lower both mole fractions, but will not change their ratio.
C. Correct. The inert gas will change the mole fractions of A and B, but the change will cancel out when calculating their ratio. End result is that no change in the number of moles of either will occur. Note that if the “ideal solution” specification wasn’t present, then this would not necessarily be true, as adding an inert could influence the fugacity coefficients of A and B.
D. Incorrect. Only one of the three options is possible.
E. Incorrect. This is true if the inert gas is being added to a constant volume vessel, but there is nothing unrealistic about maintaining constant T and P while allowing V to expand as gas is added.
You might also like to view...
Identify and state the historical significance of Thomas Jefferson.
What will be an ideal response?
Refer to the figure below. With the fork tips extended forward 20 feet and at a height of 25 feet, the maximum amount of weight the forklift can safely handle is _____.
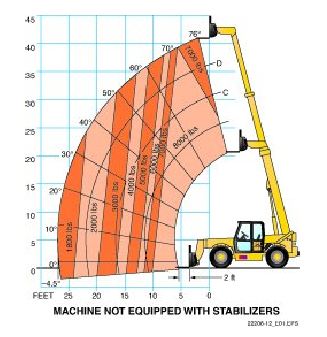
a. 1,800 pounds
b. 2,000 pounds
c. 3,000 pounds
d. 4,000 pounds
What is a chemical called that is used to kill insects?
What will be an ideal response?
In the aftermath of the Boxer uprising, the United States used the indemnity that China was forced to pay to
A. educate Chinese students in the United States. B. maintain the Open Door policy. C. establish permanent American military bases in China. D. assist the Chinese Nationalists in the efforts to overthrow the emperor. E. support U.S. missionaries in China.