In the diagram, which of the transitions would absorb a photon with the greatest energy (shortest wavelength)?
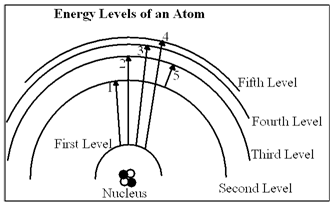
a. Transition 1
b. Transition 2
c. Transition 3
d. Transition 4
e. Transition 5
D
You might also like to view...
Photons of light can be absorbed by an atom of an element ____
a. if they match one of several possible wavelengths that are absorbed by that element b. if they match one of several possible wavelengths that are absorbed by all elements c. if they match the only particular wavelength that can be absorbed by that element d. if they match the only particular wavelength that can be absorbed by all elements e. no matter what wavelength they have
What is the "water hole"? Why is it so promising to radio astronomers?
What will be an ideal response?
A gamma ray is also known as
A) an electron. B) a positron. C) a helium nucleus. D) a high-energy photon.
Which of these is true about the absolute temperature of an ideal gas?
a. It is directly proportional to the average speed of the gas particles. b. It is directly proportional to the average kinetic energy of the gas particles. c. It does not depend on the average kinetic energy of the gas particles. d. It is directly proportional to the volume of the gas particles. e. It is directly proportional to the pressure of the gas particles.