10 moles of an ideal gas is confined in a piston-cylinder device. The gas is initially at T = 300 K and P = 1 bar. It is compressed isothermally to 10 bar. The gas has a heat capacity CV = (5/2)R.
A) What is the final volume of the gas?
B) How much work is required to accomplish the compression?
C) What is the change in internal energy of the ideal gas?
D) What is the change in enthalpy of the ideal gas?
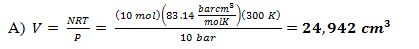
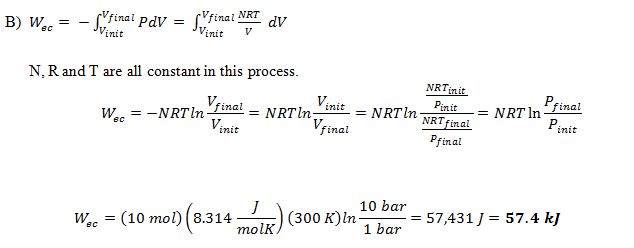

Here temperature is constant. Change in internal energy is 0.
dH ?= C_P dT for any ideal gas process, whether constant pressure or not.
Here temperature is constant. Change in enthalpy is 0.
You might also like to view...
Answer the following statement(s) true (T) or false (F)
1. The triac is a PNPN junction connected in series with an NPNP junction. 2. The triac has only two states of operation, on or off. 3. The triac is used as the input of many solid-state relays. 4. The triac can be used to control an AC voltage. 5. The triac, like the SCR, must be phase shifted if complete voltage control is to be obtained.
What were the benefits of hiring unemployed artists and writers through the Federal Art Project of the Works Progress Administration?
What will be an ideal response?
The role of a manager in which customers see him or her as speaking on behalf of the company is ______
A) Judge B) Conduit C) Position holder D) All of the above
A(n)____________________ will shutdown a process but does not completely remove the equipment from the power supply.
A. disconnect B. pushbutton C. breaker D. fuse