Why does oxygen have such a low solubility in water?
A. Water's attraction for itself is stronger than its attraction for oxygen molecules.
B. Water and oxygen only attract one another by means of weak dipole-induced dipole attractions.
C. The hydrogen bonding in water keeps the oxygen solubility low.
D. Both A and B are true.
Answer: D
You might also like to view...
On this map of South America and adjacent areas, which site would have earthquakes along a subduction zone?
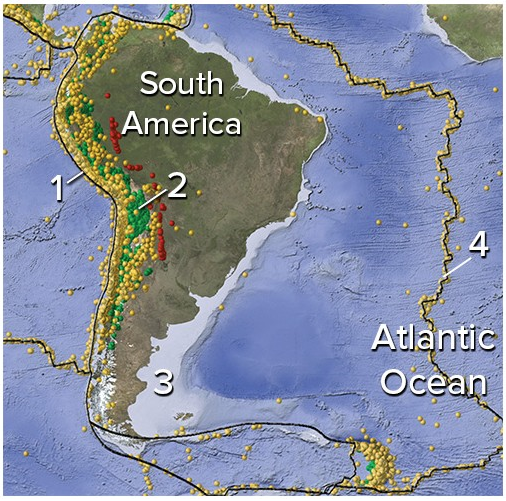
A) 1, the oceanic trench
B) 2, below the magmatic belt
C) 3, along a passive margin
D) 4, in the middle of the Atlantic Ocean
E) Both 1 and 2.
More sunlight reaches Earth's surface at the equator than at the poles.
Answer the following statement true (T) or false (F)
True/False: We expect sinking motion and clear skies to be associated with a surface high-pressure system.
a. True b. False
When the source of pollutant is readily identified by release at one location then the type of pollution is called
A. nonpoint source. B. point source. C. leachate. D. All the choices are correct.