Radioactive Decay: The half-life of cobalt-60 is 5.3 years, while that of strontium-90 is about 29 years. Suppose you have samples of both isotopes, and that they initially have the same activity (number of decays per second). What must be true of the numbers of cobalt-60 and strontium-90 nuclei in these samples?
A. There are more strontium-90 than cobalt-60 nuclei.
B. There are equal numbers of cobalt-60 and strontium-90 nuclei.
C. There are more cobalt-60 than strontium-90 nuclei.
D. It is not possible to compare numbers of nuclei without knowing the masses of the samples.
Answer: A
You might also like to view...
Astronomers believe that the hydrogen atoms in your body were created
a. in the planets. b. inside the stars. c. in the Sun. d. in the big bang. e. in neutron stars.
A system consisting of a quantity of ideal gas has the two isotherms shown. The system, initially at state C, can be taken along path C-A to final state A or along path C-B to state B
Which of the following is true?
Exhibit 17-1
The figure below shows a sine wave at one point of a string as a function of time.
?
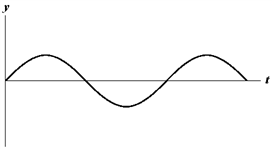
?
Use the exhibit to answer the following question(s).
Refer to . Which of the graphs below shows a wave where the amplitude and the frequency are doubled?
a.
What is the equivalent resistance between points a and b when R = 12 ??
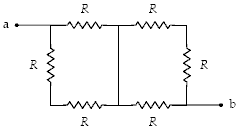
a.
20 ?
b.
16 ?
c.
24 ?
d.
28 ?
e.
6.0 ?