Choose the correct statement:
a. A pH 6.3 solution has 100 times more H+ ions per liter than a pH 4.3 solution.
b. 10 ml of a pH 6.3 solution has 10 times fewer H+ ions than 10 ml of a pH 4.3 solution.
c. 100 ml of a solution at pH 6.3 has 10 times more H+ ions per liter than 10 ml of a solution at 6.3
d. A solution at pH 7.35 has 10 times more H+ ions per liter than a solution at pH 7.45.
e. 10 ml of a pH 7.45 solution has the same H+ ion concentration as 1 ml at pH 7.45.
E
You might also like to view...
Compartments A and B are separated by a membrane that is permeable to K+ but not to Na+ or Cl-. At time zero, a solution of KCl is poured into compartment A and an equally concentrated solution of NaCl is poured into compartment B. Which would be true once equilibrium is reached?
A. Diffusion of K+ from A to B will be greater than the diffusion of K+ from B to A. B. The electrical potential difference and diffusion potential due to the concentration gradient for K+ will be equal in magnitude and opposite in direction. C. The concentration of Cl- will be higher in B than it was at time zero. D. The concentration of Na+ in A will be higher than it was at time zero. E. There will be a potential difference across the membrane, with side B negative relative to side A.
Using Figure 17.3, match the following:
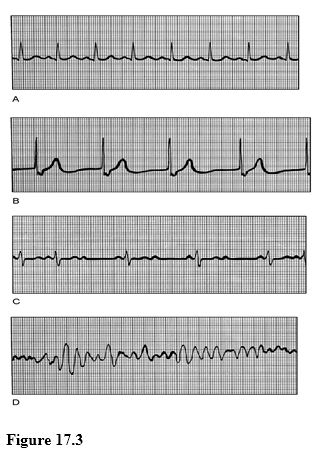
1) Ventricular fibrillation.
2) Second-degree heart block.
3) Junctional rhythm.
4) Normal sinus rhythm.
For a blood pressure reading of 136/78, the 78 refers to the pressure in the major arteries when the heart's ventricles are relaxing
Indicate whether the statement is true or false
Cardiac muscle is able to use a variety of fuel types for cellular respiration.
Answer the following statement true (T) or false (F)