Solve the problem.The pressure of a gas varies jointly as the amount of the gas (measured in moles) and the temperature and inversely as the volume of the gas. If the pressure is 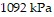 (kiloPascals) when the number of moles is 7, the temperature is
(kiloPascals) when the number of moles is 7, the temperature is 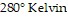 , and the volume is
, and the volume is 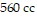 , find the pressure when the number of moles is 9, the temperature is
, find the pressure when the number of moles is 9, the temperature is 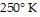 , and
, and
the volume is  .
.
A. 1352
B. 1300
C. 624
D. 650
Answer: D
You might also like to view...
Convert the angle to decimal degree notation. Round your answer to two decimal places.-62°47'13''
A. -62.83° B. -62.79° C. -62.78° D. -62.73°
Solve the problem.The half-life of a certain radioactive substance is 5 years. Suppose that at time  there are 28 g of the substance. Then after t years, the number of grams of the substance remaining will be N(t) = 28
there are 28 g of the substance. Then after t years, the number of grams of the substance remaining will be N(t) = 28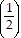 t/10How many grams of the substance will remain after 45 years?Round to the nearest hundredth of a gram.
t/10How many grams of the substance will remain after 45 years?Round to the nearest hundredth of a gram.
A. 1.24 g B. 0.15 g C. 0.62 g D. 0.31 g
Write an equation that expresses the statement. y varies inversely as G.
What will be an ideal response?
Approximate the area of the triangle to the nearest tenth.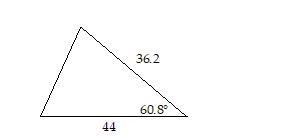
A. 691 B. 710.3 C. 695.2 D. 704.5