A rubber object is in contact with nitrogen (N2) at 298 K and 250 kPa. The solubility of nitrogen gas in rubber is 0.00156 kmol/m3?bar. The mass density of nitrogen at the interface is
(a) 0.049 kg/m3
(b) 0.064 kg/m3
(c) 0.077 kg/m3
(d) 0.092 kg/m3
(e) 0.109 kg/m3
(e) 0.109 kg/m3
T=298 [K]
P_N2_gasside=250 [kPa]*Convert(kPa, bar)
S=0.00156 [kmol/m^3-bar] "Table 14-7"
C_N2_solidside=S*P_N2_gasside
M_N2=MolarMass(N2)
rho_N2_solidside=C_N2_solidside*M_N2
You might also like to view...
____________________ are two (or more) diameters that are shown on a drawing as being on the same center line.
What will be an ideal response?
List the important aspects associated with Ai and AA-402.
What will be an ideal response?
The small amount of refrigerant released when removing gauge hoses from the system is called ____________________.
Fill in the blank(s) with the appropriate word(s).
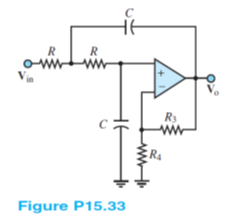
A low-pass Sullen and Key filter is shown in Figure P15.33. Find the voltage gain Vo/Vi as a function of frequency and generate its Bode magnitude plot. Show and observe that the cutoff frequency is 1/2 ?RC and that the low-frequency gain is R4/R3.