Using both the ideal gas law and van der Waals equation, determine the pressure of N2 that has a specific volume of 0.95 m3/kg and a temperature of 350 K.
Given: N2, v = 0.95 m3/kg; T = 350 K.
What will be an ideal response?
For N2, R = 0.2968 kJ/kg-K
From the ideal gas law: P = RT/v = 109.3 kPa
For van der Waals Equation, we need the constants a and b:
First, for N2, Tc = 126.2 K, Pc = 3390 kPa
Then, a = 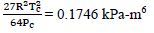
b = 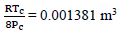
From van der Waals equation: 
You might also like to view...
The process of identifying the location of a specific gene on a chromosome is called ____________________
Fill in the blank(s) with the appropriate word(s).What permits designs to be judged as effective solutions to problems rather than just personal expressions of preference?
a. opinions c. licensing b. experts d. principles
A power steering pressure switch sends a signal to the powertrain control module, indicating higher engine speed is needed during sharp cornering at slow speeds
Indicate whether the statement is true or false
Which of the following items do aluminum conductors require an application of during installation?
A) Antioxidation paste B) Iron C) Nickel D) Oxidation paste