The conditions of a gas change in a steady-flow process from 20°C and 1000 kPa to 60°C and 100 kPa. Devise a reversible nonflow process (any number of steps) for accomplishing this change of state, and calculate ?U and ?H for the process on the basis of 1 mol of gas. Assume for the gas that PV/T is constant, 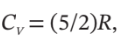 and
and 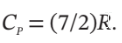
What will be an ideal response?
This is a two-step process, going first from 20°C to 60°C, and then 1000 kPa to 100 kPa. So for temperature: 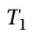 = 293.16 K and
= 293.16 K and 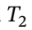 = 333.16 k , and using
= 333.16 k , and using 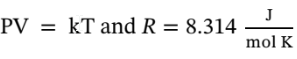 to determine the intermediate temperature and the volume changes we have
to determine the intermediate temperature and the volume changes we have
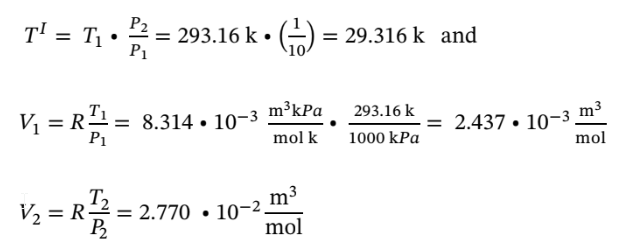
Using the intermediate temperature to determine the temperature change in both steps we have
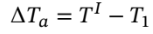 = ?263.844 k and
= ?263.844 k and 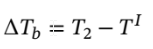 = 303.844 k.
= 303.844 k.
Using =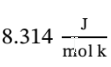 , and plugging the temperatures into we have for Step A:
, and plugging the temperatures into we have for Step A:
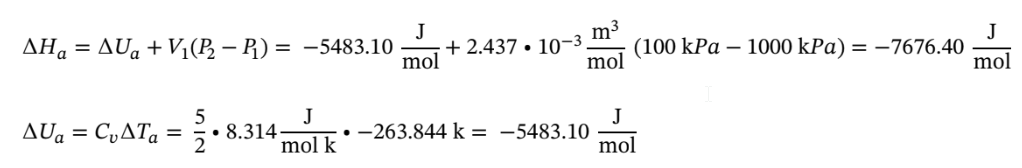
And for Step B:
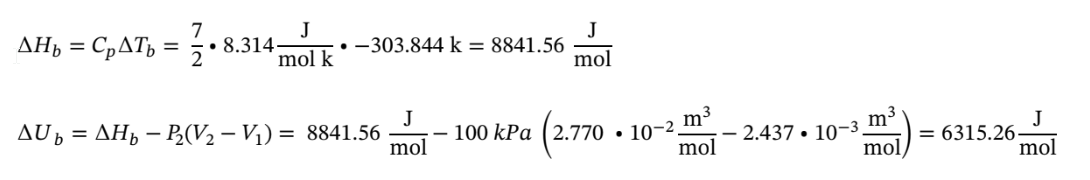
Now using these to determine the total ?U and ?H
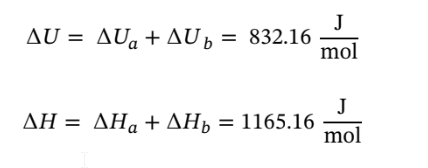
You might also like to view...
Explain what is meant by the term "forcing" and explain how it is done
What will be an ideal response?
Coal, oil, and natural gas are all examples of:
A) Renewable energy sources. B) Non-renewable energy sources. C) Perpetual energy sources. D) Low carbon energy sources.
Which of the following logic expressions represents the logic diagram in Figure 5-1?
A) X = AB + AB B) X = AB + A B C) X = AB + AB D) X = A B + AB
 The accompanying figure illustrates the steps for drawing an arc. Describe step 4.
The accompanying figure illustrates the steps for drawing an arc. Describe step 4.
What will be an ideal response?