A computer chip with a mass of 50 g, made of copper, experiences an electrical work input of 10-W. The chip is insulated. Initially, the chip has a temperature of 20oC. What is the temperature of the chip after one minute of operation?
Given: Cu; m = 50 g = 0.050 kg; W?= -10 W = -0.010 kW; T1 = 20oC; t = 1 min = 60 s
Assume: Q = 0 (insulated); ?KE = ?PE = 0
What will be an ideal response?
Solution: This is a closed system. Therefore, beginning with the First Law for closed systems, the First Law can be reduced to
W = m (u1 – u2)
In one minute, the total work is W = W?t = (-0.01 kW) (60 s) = -0.60 kJ
Treat the copper as an incompressible substance with constant specific heats: c = 0.385 kJ/kg-K
W = m c (T1 – T2)
Solving, T2 = 51.2oC
Clearly, heat must be transferred away from the chip, or the temperature would quickly rise to the point of damaging/melting the chip.
You might also like to view...
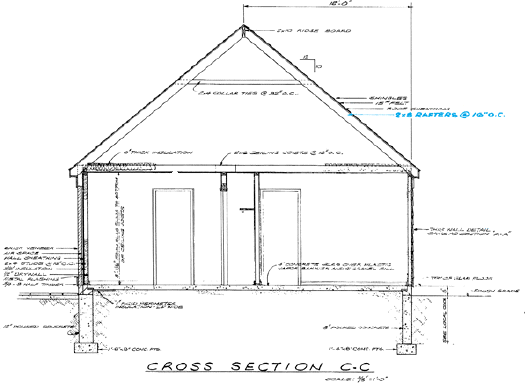
A. roof framing plan B. section view of the roof C. rafter table D. general roof plan view
Headlights can be aimed by using ________
A) An aiming screen B) A bubble level C) Either A or B D) Neither A nor B
What is the term for ladders that are designed to be removed from the apparatus?
a. pompier ladders b. bangor ladders c. articulating ladders d. portable ladders.
What two types of dial indicators can be used to measure rotor runout?
What will be an ideal response?