A cylinder contains 23 moles of an ideal gas at a temperature of 300 K. The gas is compressed at constant pressure until the final volume equals 0.43 times the initial volume
The molar heat capacity at constant volume of the gas is 24.0 J/mol • K and the ideal gas constant is R = 8.314 J/mol ? K. The heat absorbed by the gas is closest to
A) -130 kJ.
B) -94 kJ.
C) 130 kJ.
D) 94 kJ.
E) -33 kJ.
A
You might also like to view...
Addition by 1. Components: The figure shows four vectors, 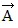 ,
, 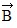 ,
, 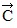 , and
, and 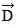 . Vectors
. Vectors 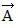 and
and 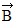 each have a magnitude of 7.0 cm, and
each have a magnitude of 7.0 cm, and
vectors 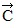 and
and 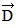 each have a magnitude of 4.0 cm. Find the x and y components of the sum of these four vectors.
each have a magnitude of 4.0 cm. Find the x and y components of the sum of these four vectors.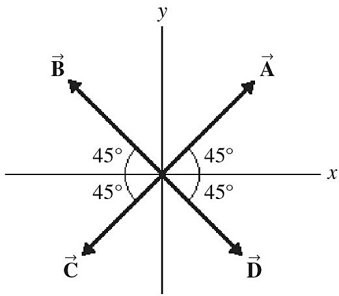
What will be an ideal response?
Mort observes Velma's light-clock, as Velma passes. He observes that, during a 1 second tick of her clock, her light beam travels
A) 300,000 km. B) a distance of one light year. C) less than 300,000 km. D) further than 300,000 km. E) no distance at all.
The law of conservation of strangeness tells us that strangeness is not conserved for which of the following interactions?
a. strong b. electromagnetic c. weak d. All three of the above interactions conserve strangeness.
A hot piece of iron is thrown into the ocean and its temperature eventually stabilizes. Which of the following statements concerning this process is correct? (There may be more than one correct choice.)
A) The change in the entropy of the iron-ocean system is zero. B) The entropy gained by the iron is equal to the entropy lost by the ocean. C) The entropy lost by the iron is equal to the entropy gained by the ocean. D) The ocean gains more entropy than the iron loses. E) The ocean gains less entropy than the iron loses.