Solve the problem.The pressure of a gas varies jointly as the quantity of the gas (measured in moles) and the temperature and inversely as the volume of the gas. Suppose that the pressure of a particular gas is 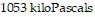 (kPa) when the quantity is
(kPa) when the quantity is 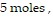 the temperature is
the temperature is 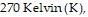 and the volume is
and the volume is  Find the pressure of that gas when the number of moles is 7, the
Find the pressure of that gas when the number of moles is 7, the
temperature is  and the volume is
and the volume is 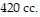
A. 1716 kPa
B. 910 kPa
C. 1768 kPa
D. 884 kPa
Answer: C
Mathematics
You might also like to view...
Factor completely.5x2 - 30x + 45
A. (5x - 15)(x - 3) B. 5(x - 9)(x + 1) C. (x - 3)(5x - 15) D. 5(x - 3)(x - 3)
Mathematics
Give the coordinates of the point described on the unit circle.The reflection of the point 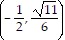 across the origin
across the origin
A. 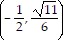
B. 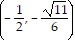
C. 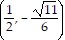
D. 
Mathematics
Find the largest open interval where the function is changing as requested.Increasing f(x) = 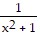
A. (0, ?) B. (-?, 0) C. (1, ?) D. (-?, 1)
Mathematics
Evaluate the integral.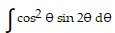
A. - 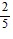 cos5 ? + C
cos5 ? + C
B. - 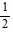 cos4 ? + C
cos4 ? + C
C. 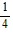 cos3 ? + C
cos3 ? + C
D. 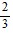 cos2 ? + C
cos2 ? + C
Mathematics