For each of the original chemical compositions and temperatures at 1 atmosphere of pressure for the aluminum (Al)-nickel (Ni) alloys (see Figure 5.15) listed below, give the following:
1. The phases present.
2. From the Gibbs phase rule, determine the degrees of freedom in the material. Can the composition of the phases or phases present be adjusted by changing the original chemical composition?
3. The chemical composition of each phase.
4. The atom fraction of each phase.
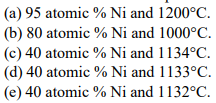
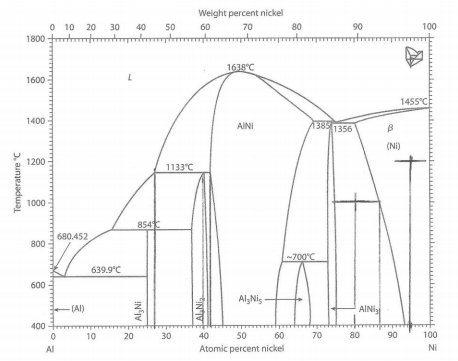
a.1 The point 95 atomic % Ni and 1200?C is in the single phase region of Ni. Ni in this phase diagram is the FCC Ni crystal structure with substitutional atoms of Al.
a.2 The GPR is P + F = C + 1, 1 + F = 2 + 1, F=2. The temperature and composition can be set for the single phase for F = 2.
a.3 In the single phase region of Ni, CNi = 0.950 atoms of Ni in Ni/total atoms. a.4 fNi = 1, or 100% of the atoms are in Ni.
b.1 80Ni-20Al and 1000?C is in the two phase region of Ni and AlNi3
In this phase diagram only the single phase regions are labeled. Areas between single phase regions will always be two phase regions made up of the two single phases on either side of the two phase region.
b.2 P = 2, therefore the GPR is 2 + F = 2 + 1 and F =1 and that one choice is temperature. There is no possible choice in composition.

You might also like to view...
Pluto is no longer considered a planet
Indicate whether the statement is true or false
Is the back side of the Moon always dark?
What will be an ideal response?
What current flows in a 60. mH inductor when 12. V AC at a frequency of 20. kHz is applied to it?
What will be an ideal response?
What is the approximate diameter of Earth in miles given the information provided in the figure caption: "Earth spills over the edges of this photograph at a scale of 10^7 meters"?
a. 10,000 mi b. 6,000 mi c. 3,000 mi d. 1,000 mi