Solve the problem.The hydrogen potential, pH, of a substance is defined by 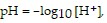 where the hydrogen ion concentration, [H+], is measured in moles per liter. Find the hydrogen ion concentration of a solution whose pH is 7.4.
where the hydrogen ion concentration, [H+], is measured in moles per liter. Find the hydrogen ion concentration of a solution whose pH is 7.4.
A. 2.51 × 107 moles per liter
B. 3.98 × 10-8 moles per liter
C. 0.86923172 moles per liter
D. -0.8692317 moles per liter
Answer: B
Mathematics
You might also like to view...
Find the vertex of the parabola.f(x) = 2x2 - 12x + 3
A. (2, -3) B. (3, -15) C. (-2, 3) D. (-3, 2)
Mathematics
Find the exact value of the expression.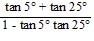
A. 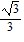
B. 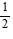
C. 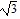
D. 2
Mathematics
Perform the indicated row operations on the following matrix. 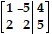 (-2)R1 + R2 ? R2
(-2)R1 + R2 ? R2
A. 
B. 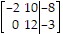
C. 
D. 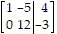
Mathematics
Simplify the expression without using a calculator.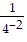
A. - 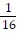
B. - 16
C. 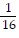
D. 16
Mathematics