The energy of the ground state in the Bohr model of the hydrogen atom is -13.6 eV. The energy of the n = 2 state of hydrogen in this model is closest to
A) -4.5 eV. B) -6.8 eV. C) -1.7 eV. D) -13.6 eV. E) -3.4 eV.
E
You might also like to view...
An L-shaped beam is loaded as shown in the figure. The bending moment at the midpoint of span AB is approximately:
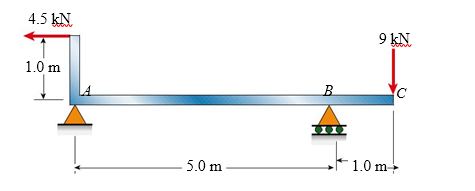
(A) 6.8 kN?m
(B) 10.1 kN?m
(C) 12.3 kN?m
(D) 15.5 kN?m
A hydrogen atom emits a photon as it makes a transition from the n = 4 state to the n = 3 state. The energies of these two states are –0.9 eV and –1.5 eV, respectively. (a) What is the energy of the photon? (b) What is its frequency?
If the voltage at a point in space is zero, then the electric field must be
A) negative. B) zero. C) uniform. D) positive. E) impossible to determine based on the information given.
On a cold day, a heat pump absorbs heat from the outside air at 14°F (?10°C) and transfers it into a home at a temperature of 86°F (30°C). Determine the maximum ? of the heat pump
a. 0.2 b. 4.4 c. 0.5 d. 7.6 e. 6.7