Light shines through atomic Hydrogen gas. It is seen that the gas absorbs light readily at a wavelength of 91.33 nm
What is the level to which the Hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level. A) 25
B) 27
C) 22
D) 32
A
You might also like to view...
Instructions: On occasion, the notation 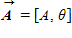
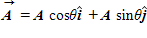
If 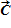
Which of these particles has the least mass?
a. electron neutrino b. muon c. electron d. pion
A velocity selector uses a fixed electric field of magnitude E and the magnetic field is varied to select particles of various energies. If a magnetic field of magnitude B is used to select a particle of a certain energy and mass, what magnitude of magnetic field is needed to select a particle of equal mass but twice the energy?
a. 0.50 B b. 1.4 B c. 2.0 B d. 0.71 B e. 1.7 B
The alternating current in wide use in homes in North America is 60 cycles per second, or 60 Hz. How many times per minute does this current reverse its direction of travel?
A. 3600 times B. 60 times C. 7200 times D. 120 times E. None of these choices are correct