You are given samples of PVC, exposed to warm cooking oil (90 °F) and acetone. Based on polymer structure, what would you expect to happen to the samples? Why?
What will be an ideal response?
In the polymer structure of polystyrene there is a pendent chlorine atom. This not only prevents the polymer from close packing but also makes it solvent sensitive. The samples would swell with the elevated temperature or water and the polarity of acetone. The free chlorine bonds to the polar water or acetone. The cooking oil wouldn't have this effect as it isn't a polar substance.
You might also like to view...
The ____________________ is a brushless DC, three-phase motor with a permanent magnet rotor.
Fill in the blank(s) with the appropriate word(s).
A customer complains that every time the lights are turned on in the vehicle, the dash display dims. Which of these is the MOST likely cause which of these conditions?
A) Normal behavior for VTF dash display B) Poor ground in lighting circuit causing a voltage drop to dash lamps C) Normal behavior for LED dash displays D) Feedback problem from a short-to-voltage between headlights and dash display
What is the c-c of pipe M?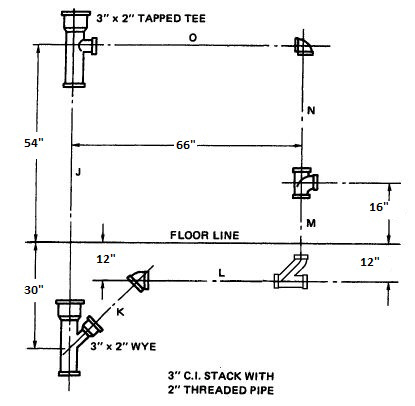
Fill in the blank(s) with the appropriate word(s).
What is the primary job of the PMD sublayer?
A. translate binary 1s and 0s into radio signals B. evaluate the Header error check field C. reformat the data received from the MAC layer D. implement a channel access method