Consider that the compartments of the insulated box of Prob. 7.5 initially contain on one side a mole of ideal gas A and on the other side a mole of ideal gas B, with both gases at a pressure of 0.1013 MPa and a temperature of 298 K. Now assume that the partition is removed.
(a) How large will the enthalpy change be?
(b) Compute the entropy change.
(c) Compute the Gibbs free?energy change. Is the magnitude of this change significant? Explain.
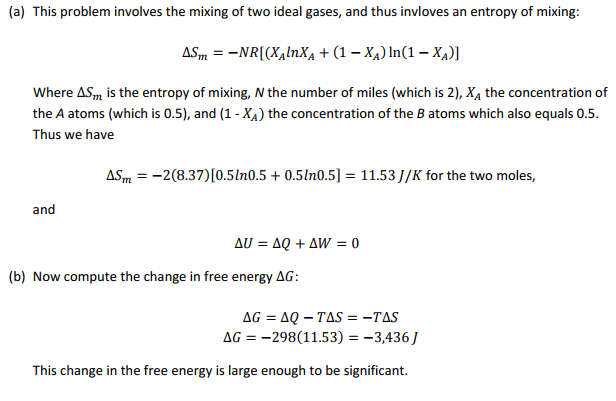
You might also like to view...
In a high-rise building, the concrete may be brought from the ground to the location of placement by
A) chutes. B) pumping. C) buckets. D) A or B. E) B or C.
Sand filters in pools remove which of the following?
a. bacteria b. viruses c. solids d. algae
When is the O terminal energized on most low-voltage thermostats?
A) Whenever the thermostat calls for cooling B) Whenever the system switch is moved to "Heat" C) Whenever the thermostat calls for first stage heat D) Whenever the system switch is moved to "Cool"
There is no air flow through the PCV valve when the engine is idling.
Answer the following statement true (T) or false (F)