How would the values of vp for carbon dioxide gas compare to that of nitrogen gas at a given temperature?
A. vp will be larger for carbon dioxide.
B. vp will be larger for nitrogen.
C. vp will be equal in both cases.
D. One does not have enough information to tell for sure.
B. vp will be larger for nitrogen.
You might also like to view...
Which of the following is the main form of intermolecular attractions among water molecules?
A) hydrogen bonding B) induced dipole-induced dipole C) covalent bonding D) ion-dipole E) polar-induced polar
What is the CNO cycle?
A) the process by which helium is fused into carbon, nitrogen, and oxygen B) the process by which carbon is fused into nitrogen and oxygen C) the set of fusion reactions that have produced all the carbon, nitrogen, and oxygen in the universe D) a set of steps by which four hydrogen nuclei fuse into one helium nucleus
The circuit below contains 4 100-W light bulbs. The emf is 110 V. Which light bulb(s) is(are) brightest?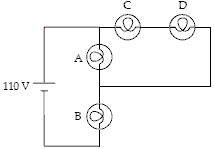
A. A B. B C. C D. D E. C and D
Which of the following does quantum theory agree with?
A) Microscopic particles, rather than the macroscopic world, form the fundamental reality. B) Future events are entirely predetermined, or predictable. C) It is possible to analyze physical phenomena and physical systems by separating them into their component parts and studying those parts individually. D) All of the above. E) None of the above.