Solve the problem.The pH of a solution is defined as pH = -log[H+], where [H+] is the concentration of hydrogen ions in the solution. The pH of pure water is 7, while the pH of vinegar is about 3. How much greater is the concentration of hydrogen ions in vinegar than in pure water?
A. 1,000,000 times greater
B. 4 times greater
C. 10,000 times greater
D. 1000 times greater
Answer: C
You might also like to view...
Solve the problem.The surface area of a hollow cylinder (tube) is given by 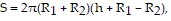 where h is the length of the cylinder and R1 and R2 are the outer and inner radii. If h, R1, and R2 are measured to be 4 inches, 7 inches, and 9 inches respectively, and if these measurements are accurate to within 0.1 inches, estimate the maximum percentage error in computing S.
where h is the length of the cylinder and R1 and R2 are the outer and inner radii. If h, R1, and R2 are measured to be 4 inches, 7 inches, and 9 inches respectively, and if these measurements are accurate to within 0.1 inches, estimate the maximum percentage error in computing S.
A. 0.084% B. 0.128% C. 0.106% D. 0.172%
Find the indicated series by the given operation.Show that by differentiating term by term the expansion of 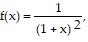 the result is the expansion for
the result is the expansion for 
A.  (1 + 2x - 3x2 + 4x3 - . . .) = -2(1 + 3x - 6x2 + . . . )
(1 + 2x - 3x2 + 4x3 - . . .) = -2(1 + 3x - 6x2 + . . . )
B.  (1 - 4x + 6x2 - 8x3 + . . .) = -2( 2 - 6x + 12x2 - . . .)
(1 - 4x + 6x2 - 8x3 + . . .) = -2( 2 - 6x + 12x2 - . . .)
C.  (1 - 2x + 3x2 - 4x3 + . . .) = -2(1 - 3x + 6x2 - . . .)
(1 - 2x + 3x2 - 4x3 + . . .) = -2(1 - 3x + 6x2 - . . .)
D. 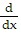 (2x + 3x2 - 4x3 + . . .) = -2(1 - 3x + 6x2 - . . .)
(2x + 3x2 - 4x3 + . . .) = -2(1 - 3x + 6x2 - . . .)
Solve for y or k, as appropriate.ln (y - 35) = 4x
A. e4x + 35
B. 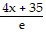
C. 4x + 35
D. ln (4x) + 35
Provide an appropriate response. Identify the equation y = -3x2 + 1 as linear, quadratic, or other.
A. other B. quadratic C. linear