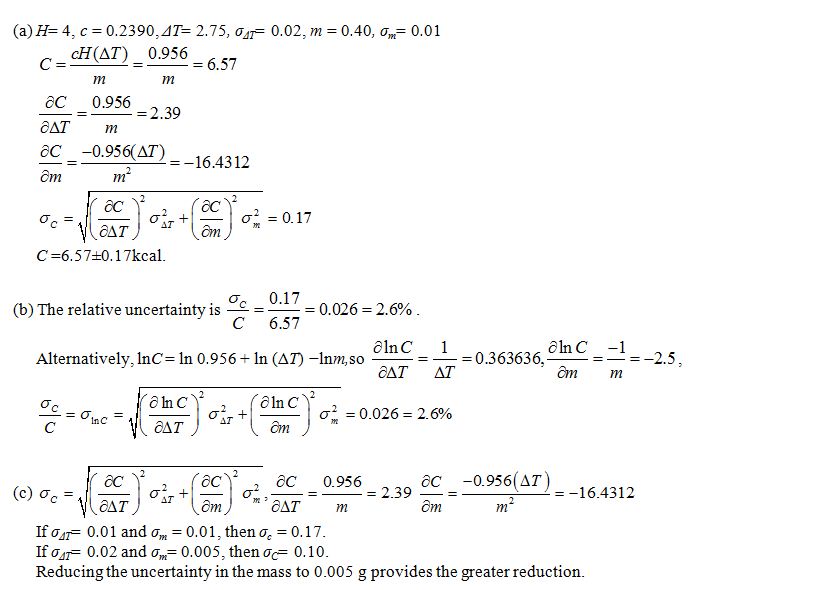
The heating capacity of a calorimeter is known to be 4 kJ/°C, with negligible uncertainty. The number of dietary calories (kilocalories) per gram of a substance is given by C=cH(?T)/m, where C is the number of dietary calories,H is the heating capacity of the calorimeter, ?T is the increase in temperature in °C caused by burning the substance in the calorimeter, m is the mass of the substance in grams, and c=0.2390 cal/kJ is the conversion factor from kilojoules to dietary calories. An amount of mayonnaise with mass 0.40 ± 0.01 g is burned in a calorimeter. The temperature increase is 2.75 ± 0.02°C.
a. Estimate the number of dietary calories per gram of mayonnaise, and find the uncertainty in the estimate.
b. Find the relative uncertainty in the estimated number of dietary calories.
c. Which would provide a greater reduction in the uncertainty in C: reducing the uncertainty in the mass to 0.005 g or reducing the uncertainty in ?T to 0.01°C?
You might also like to view...
A specialized stem that emerges from the axil of a leaf, grows horizontally along the ground and produces new plantlets at every other node is known as a ________.
Fill in the blank(s) with the appropriate word(s).
The dairy breed that produces milk with the highest fat content is the _____
a. Jersey c. Brown Swiss b. Guernsey d. Holstein
The first phase of Hidden Valley includes ____ buildings.
A. nine B. ten C. eleven D. twelve
The ________ is a unit of electrical pressure
A) Coulomb B) Ampere C) Ohm D) Volt