Assume the Heisenberg uncertainty principle can take the form ?E?t ? h/4B. How accurate can the position of an electron be made if its speed is 5 × 10^6 m/s and if the uncertainty in its energy is 10 eV?
a. 7.7 × 10^?18 m
b. 1.3 × 10^?23 m
c. 6.2 × 10^?15 m
d. 1.6 × 10^?10 m
e. 1.2 × 10^?9 m
d
You might also like to view...
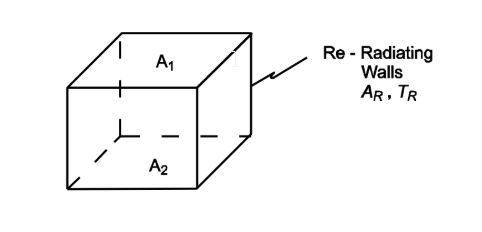
Show that the effective conductance. A1 F 1-2 for two black parallel plates of equal area connected by re-radiating walls at a constant temperature is

GIVEN
• Two black parallel planes of equal area connected by re-radiating walls at a constant temperature
FIND
• Show that

SKETCH
For a 10 mol mixture of air (8 mols nitrogen, N2, and 2 mols oxygen, O2) at 101 kPa, 20ºC, determine the mass concentration and the molar concentration of N2 and O2.
What will be an ideal response?
What is the importance of the carbon dioxide (CO2) cycle?
A) It regulates the carbon dioxide concentration of our atmosphere, keeping temperatures moderate. B) It makes the growth of continents possible. C) It allows for an ultraviolet-absorbing stratosphere. D) It will prevent us from suffering any consequences from global warming.
What is the power output of a hot air furnace that produces heat at the rate of 160,000. BTU/hr?
A) 22 kW B) 80 kW C) 47 kW D) 7.6 kW E) 1.6 kW