Calculate the rate of heat transfer between a 15-cm-OD pipe at 120°C and a 10-cm-OD pipe at 40°C. The two pipes are 330-m-long and are buried in sand [k = 0.33 W/(m K)] 12 m below the surface (Ts = 25°C). The pipes are parallel and are separated by 23 cm (center to center) distance.
GIVEN
Two parallel pipes buried in sand
Pipe 1
Outside diameter (D1) = 15 cm = 0.15 m
Temperature (T1) = 120°C
Pipe 2
Outside diameter (D2) = 10 cm = 0.1 m
Temperature (T2) = 40°C
Length of pipes (L) = 330 m
Thermal conductivity of the sand (k) = 0.33 W/(m K)
Depth below surface (d) = 1.2 m
Surface temperature (Ts) = 25°C
Center to center distance between pipes (s) = 23 cm = 0.23 m
FIND
The rate of heat transfer between the pipes (q)
ASSUMPTIONS
The thermal conductivity of the sand is uniform
Two dimensional, steady state heat transfer
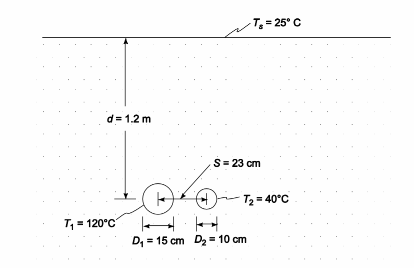
For the pipe-to-pipe heat transfer, the surface is not important since Z >> D. The shape factor for this
geometry,
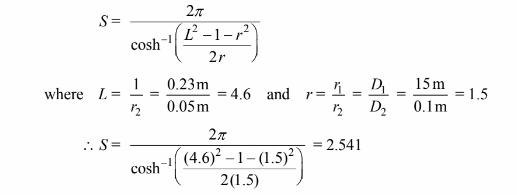
The rate of heat transfer per unit length is

You might also like to view...
What is the wavelength of the line in the Balmer series of hydrogen that is comprised of transitions from the n = 5 to the n = 2 level? (R = 1.097 × 10^7 m?1 and 1 nm = 10?9 m)
a. 304 nm b. 434 nm c. 476 nm d. 95 nm e. 333 nm
What is the ratio of the light-gathering power of a 5-m telescope to that of a 0.5-m telescope?
a. 10 b. 0.1 c. 0.01 d. 100 e. 25
Compton Scattering: In a Compton scattering experiment using x-rays, the wavelength of the x-rays increases by 5.0% as the light is scattered at an angle of 60° with its original direction. What was the original wavelength of the light before scattering? (melectron = 9.11 × 10-31 kg, c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ? s)
Fill in the blank(s) with the appropriate word(s).
If you add 1.33 MJ of heat to 500 g of water at 50°C in a sealed container, what is the final temperature of the steam?
The latent heat of vaporization of water is 22.6 × 105 J/kg, the specific heat of steam is 2010 J/kg ? K, and the specific heat of water is 4186 J/kg ? K. A) 100°C B) 112°C C) 123°C D) 147°C E) 195°C